Principle of the Procedure
The 3 Panel Oral Fluid Drug Test is a competitive immunoassay designed for the qualitative detection of selected drugs or their metabolites in oral fluid. The device operates as a chromatographic absorbent system, in which analytes present in the specimen compete with immobilized drug conjugates for a limited number of antibody–dye conjugate binding sites.
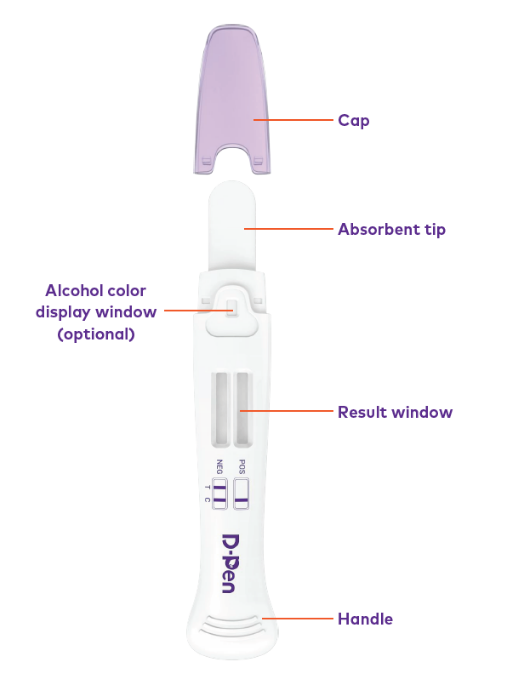
When the absorbent collection tip is placed in the oral cavity, oral fluid is drawn into the device by capillary action, where it mixes with the antibody–dye conjugate and migrates across a membrane pre-coated with drug–protein conjugates.
If the drug concentration in the specimen is below the designated cutoff level, the antibody–dye conjugate binds to the immobilized drug–protein conjugates in the Test (T) region, forming a visible colored line. Any visible line in the T region, regardless of intensity, should be interpreted as a negative result.
If the drug concentration in the specimen is at or above the cutoff level, the free drug molecules present in the sample bind preferentially to the antibody–dye conjugate. This prevents binding at the T region, and no line will appear. The absence of a line in the T region indicates a presumptive positive result.
A colored line in the Control (C) region confirms that the test has been performed correctly and that the device is functioning properly.
This assay provides rapid specimen collection, consistent fluid migration, and reduced opportunities for adulteration. It is suitable for use in forensic, employment, insurance, and reasonable suspicion testing scenarios.
Materials Provided
3 Panel Oral Fluid Drug Test device(s) (per kit)
Instructions for Use (IFU)
Materials Required but Not Provided
Timer or stopwatch
Directions for Use (Quick Reference)
Instruct the donor not to eat, drink, or smoke for at least 30 minutes prior to sample collection.
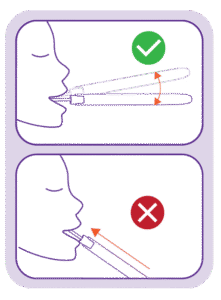
Place the absorbent collection tip in the mouth and allow oral fluid to naturally saturate the swab.
Insert the saturated swab into the device as directed in the Instructions for Use.
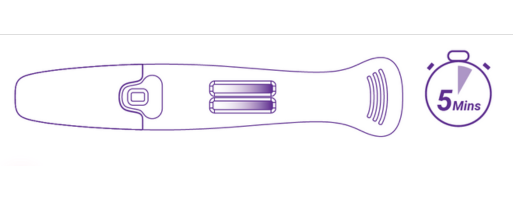
Start timing once the specimen is introduced into the device.
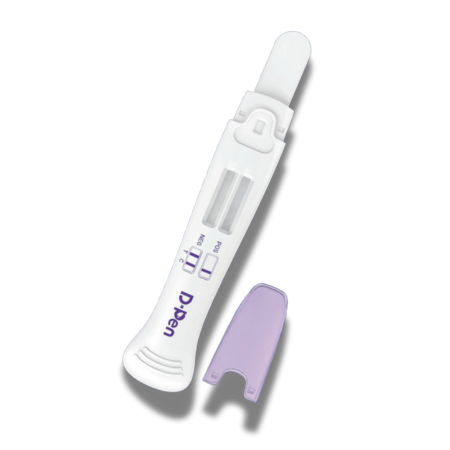
Interpretation of Results
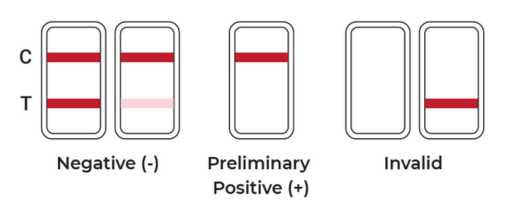
Negative: Two distinct lines appear (one at C and one at T). Any visible line at T, regardless of intensity, is considered negative.
Positive: A line appears in the C region only; no line is visible in the T region for the drug tested.
Invalid: No line appears in the C region, or lines are smeared/unclear. The test should be repeated using a new device.



















































Reviews
There are no reviews yet.